prior to EPKINLY administration

Off-the-shelf subcutaneous EPKINLY can be
administered in an outpatient setting1
Administer following the recommended 3-step up dosage schedule to reduce the incidence and
severity of CRS
EPKINLY 3-step up dosage schedule for patients with FL
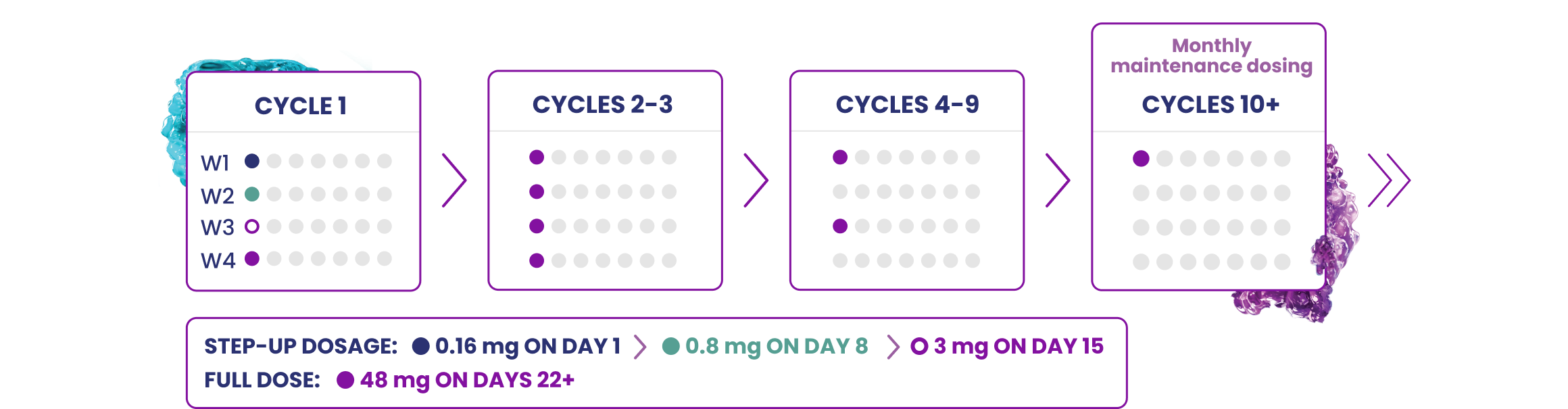
Administer EPKINLY subcutaneously in 28-day cycles to well-hydrated patients until disease progression or unacceptable toxicity.
- Prior to starting EPKINLY, provide Pneumocystis jirovecii pneumonia prophylaxis and consider initiating prophylaxis against herpes virus to prevent herpes zoster reactivation
- Premedicate before each dose in cycle 1 to reduce the incidence and severity of CRS (see Recommended Medications below)
- If a dose of EPKINLY is missed or delayed, therapy may need to be restarted (see Missed or Delayed Dose below)
Hospitalization is not required to administer EPKINLY for 3L+ FL
- EPKINLY should only be administered by a qualified HCP with appropriate medical support to manage severe reactions such as CRS and ICANS
- Due to the risk of CRS and ICANS, monitor all patients for signs and symptoms
- Hospitalization may be needed to manage select adverse reactions
EPKINLY is designed to improve tolerability and accessibility across practice settings1-4
- Subcutaneous administration allows more gradual increases and lower peaks in plasma cytokine levels than IV administration4
- EPKINLY is an off-the-shelf treatment, available to treat patients at the moment of relapse1,3
FOR 3L+ FL
Missed or delayed dose
Restarting therapy after dosage delay1
| Last dose administered | Time since last dose administered | Action for next dose(s)* |
|---|---|---|
0.16 mg (e.g., on cycle 1 day 1) |
More than 8 days |
|
0.8 mg (e.g., on cycle 1 day 8) |
More than 8 days |
|
3 mg (e.g., on cycle 1 day 15) |
14 days or less |
|
| More than 14 days |
|
|
48 mg (e.g., on cycle 1 day 22 onwards) |
6 weeks or less |
|
| More than 6 weeks |
|
Restarting therapy after dosage delay1
Last dose administered
Time since last dose administered:
More than 8 days
Action for next dose(s)*:
- Repeat cycle 1 schedule starting at step-up dose 1 (0.16 mg),
- Following the repeat of cycle 1 schedule, resume the planned treatment schedule
Time since last dose administered:
More than 8 days
Action for next dose(s)*
- Repeat cycle 1 schedule starting at step-up dose 1 (0.16 mg),
- Following the repeat of cycle 1 schedule, resume the planned treatment schedule
Time since last dose administered:
14 days or less
Action for next dose(s)*
- Administer 48 mg,
- Resume the planned treatment schedule
Time since last dose administered:
More than 14 days
Action for next dose(s)*
- Repeat cycle 1 schedule starting at step-up dose 1 (0.16 mg),
- Following the repeat of cycle 1 schedule, resume the planned treatment schedule
Time since last dose administered:
6 weeks or less
Action for next dose(s)*
- Administer 48 mg,
- Resume the planned treatment schedule
Time since last dose administered:
More than 6 weeks
Action for next dose(s)*
- Repeat cycle 1 schedule starting at step-up dose 1 (0.16 mg),
- Following the repeat of cycle 1 schedule, resume the planned treatment schedule
*Administer pretreatment medication prior to EPKINLY dose and monitor patients accordingly.
Recommended medications1
Administer medications as outlined below to reduce the risk of CRS
| Recommended pre- and post-administration medications | ||
|---|---|---|
prior to EPKINLY administration |
CYCLE 1 Administered prior to each weekly administration of EPKINLY to all patients |
CYCLES 2+ Administered prior to next administration of EPKINLY to patients who experienced grade 2 or 3‡ CRS with previous dose, until EPKINLY is given without subsequent CRS of grade 2 or higher |
| Dexamethasone† or Prednisolone or equivalent |
|
|
| Diphenhydramine or equivalent | 50 mg oral or IV, or equivalent | N/A |
| Acetaminophen | 650 mg to 1000 mg oral | N/A |
Recommended pre- and post-administration medications
CYCLE 1
Administered prior to each weekly administration of EPKINLY to all patients
Dexamethasone† or Prednisolone or equivalent
- 15 mg oral or IV (dexamethasone), 100 mg oral or IV (prednisolone), or equivalent
- And for 3 consecutive days following each weekly administration of EPKINLY in cycle 1
Diphenhydramine or equivalent
50 mg oral or IV, or equivalent
Acetaminophen
650 mg to 1000 mg oral
CYCLES 2+
Administered prior to next administration of EPKINLY to patients who experienced grade 2 or 3‡ CRS with previous dose, until EPKINLY is given without subsequent CRS of grade 2 or higher
Dexamethasone or Prednisolone or equivalent
- 15 mg oral or IV (dexamethasone), 100 mg oral or IV (prednisolone), or equivalent
- Administer prior to the next administration of EPKINLY and for 3 consecutive days following each weekly administration of EPKINLY
Diphenhydramine or equivalent
N/A
Acetaminophen
N/A
†Dexamethasone is the preferred corticosteroid when available.
‡Patients will be permanently discontinued from EPKINLY after a grade 4 CRS event.
Preparation and administration1
EPKINLY is available in 2 dosing strengths: 4 mg/0.8 mL and 48 mg/0.8 mL. Step-up doses 1 (0.16 mg) and 2 (0.8 mg) require dilution prior to administration using the EPKINLY 4 mg/0.8 mL vial by an HCP using aseptic technique. Step-up dose 3 (3 mg) is administered using the EPKINLY
4 mg/0.8 mL vial. The full dose (48 mg) is administered using a ready-to-use EPKINLY 48 mg/0.8 mL vial.
Refer to the full Prescribing Information for more detailed instructions on dose preparation and storage of EPKINLY.
Administration
- To minimize injection pain, allow EPKINLY solution to equilibrate to room temperature for no more than 1 hour before administration
- EPKINLY should be injected into the subcutaneous tissue of the lower part of the abdomen (preferred injection site) or the thigh
- Change of injection site from the left or right side or vice versa is recommended, especially during the weekly administrations (cycles 1 to 3)
- Do not inject into tattoos or scars or areas where the skin is red, bruised, tender, hard, or not intact
Watch the following video for step-by-step instructions on how to properly store, handle, and prepare EPKINLY for administration.
The purpose of this video is to provide information on EPKINLY, epcoritamab-bysp, product handling and instructions for dilution.
Before we start, it is important to understand the following.
INDICATIONS
EPKINLY is indicated for the treatment of adults with:
- relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from indolent lymphoma, and high-grade B cell lymphoma after 2 or more lines of systemic therapy.
This indication is approved under accelerated approval based on response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). - EPKINLY is indicated in combination with lenalidomide and rituximab for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL), and as monotherapy for the treatment of adult patients with relapsed or refractory FL after 2 or more lines of systemic therapy.
Please see the Important Safety Information, including Boxed Warnings for Cytokine release syndrome (CRS) and Immune effector cell-associated neurotoxicity syndrome (ICANS), and information of other warnings and precautions at the end of this video.
The recommended dosage schedules differ based on indication; so please review the recommended dosage chapter before proceeding to the dose preparation sections.
The video is divided into 8 chapters. Fast forward to the timestamps noted on the screen to review a specific chapter.
Chapter 1: Recommended Dosage and Storage & Handling
Your role in preparing EPKINLY is vital to patients and their care partners as key members of their care team.
The recommended 2 step-up dosage schedule for EPKINLY in patients with DLBCL or HGBCL includes a 0.16 mg dose as step-up dose 1, a 0.8 mg dose as step-up dose 2, followed by a 48 mg full dose for subsequent doses.
EPKINLY is administered according to the following 28-day dosing cycles until disease progression or unacceptable toxicity.
The recommended 3 step-up dosage schedule for EPKINLY in patients with FL includes a 0.16 mg dose as step-up dose 1, a 0.8 mg dose as step-up dose 2, a 3 mg dose as step-up dose 3, followed by a 48 mg full dose for subsequent doses.
EPKINLY as monotherapy for FL is administered according to the following 28-day dosing cycles until disease progression or unacceptable toxicity.
When EPKINLY is administered in combination with rituximab and lenalidomide, it follows the 3 step-up dosage schedule in cycle 1 and is then administered at the 48 mg full dose for up to 12 cycles.
Administer EPKINLY in combination with lenalidomide 20 mg on days 1 to 21 in Cycles 1 to 12 and rituximab 375 mg/m2 in Cycles 1 to 5.
Refer to the lenalidomide prescribing information and rituximab prescribing information for the respective dosage recommendations, including lenalidomide dosage recommendations for patients with renal insufficiency.
When planning the patient's treatment schedule, staff should verify availability of EPKINLY and recommended pre- and post- administration medications as detailed in the full Prescribing Information.
Herein, we will give detailed instructions on how to properly store and handle EPKINLY.
EPKINLY for subcutaneous injection is supplied in 2 single dose vials. The 4 mg/0.8 mL, single dose vial is used to prepare the step-up doses. The 48 mg/0.8 mL, single-dose vial is used to prepare the full doses
Storage of EPKINLY single-dose vials before preparation is as follows:
Store EPKINLY refrigerated at 2 to 8 degrees Celsius (36 to 46 degrees Fahrenheit)
- Do NOT freeze EPKINLY
Keep EPKINLY in the original carton to protect from light
- Do NOT shake EPKINLY
After the preparation of EPKINLY for administration: Use EPKINLY solution in the syringe immediately
- If not used immediately, store the solution
- Refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours or
- At room temperature at 20°C to 25°C (68°F to 77°F) for up to 12 hours
- The total storage time from the start of dose preparation to administration should not exceed 24 hours.
- Protect from direct sunlight
- Discard unused EPKINLY solution beyond the allowable storage time
Now that you know how to properly store and handle EPKINLY, let's go over how to properly prepare EPKINLY for administration to your patients.
EPKINLY is for subcutaneous injection only.
Certain doses of EPKINLY require dilution prior to administration.
This video will provide step-by-step instructions for safely preparing EPKINLY using two available methods: an empty sterile vial method and a sterile syringe method.
Use aseptic technique to prepare EPKINLY. Filtration of the diluted solution is not required.
Follow the preparation instructions provided in this video and the full prescribing information, as improper preparation may lead to improper dose.
Chapter 2: 0.16 mg Dose (Step-up Dose 1) EMPTY STERILE VIAL METHOD
We will begin with the empty sterile vial method.
Here we will go through the necessary steps needed to prepare the EPKINLY 0.16 mg dose using the empty sterile vial method. Step-up dose 1 is the first dose administered to patients with DLBCL, HGBCL, or FL.
Let's begin by preparing your 0.16 mg dose dilution supplies.
Here is a compilation of all supplies you will need to assemble before you get started.
Now we will go through a simulation on how to prepare a 0.16 mg dose of EPKINLY using empty sterile vials.
Use aseptic technique to prepare EPKINLY.
Filtration of the diluted solution is not required.
To prepare the 0.16 mg dose, EPKINLY 4 mg/0.8 mL requires TWO dilutions.
When diluting EPKINLY, use an appropriately sized, syringe, vial, and needle for each transfer step.
First, retrieve one 4 mg/0.8 mL EPKINLY vial (with the turquoise cap) from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
In the meantime, grab your two appropriately sized empty sterile vials and label the first empty vial “Dilution A”.
And label your second empty vial “Dilution B”.
Grab the 4 mg/0.8 mL EPKINLY vial with the turquoise cap that is now at room temperature.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Withdraw 0.8 mL of EPKINLY using an appropriately sized sterile syringe.
Transfer the 0.8 mL of EPKINLY into the Dilution A vial.
The EPKINLY vial is no longer needed and may be discarded.
REMINDER: Once you have pierced an empty vial, you have equalized the pressure within that vial, so for compounding practices, you need to make sure that you maintain negative pressure within the vial.
Grab one vial of 0.9% Sodium Chloride for injection.
Withdraw 4.2 mL of 0.9% Sodium Chloride.
Transfer 4.2 mL of 0.9% Sodium Chloride into the Dilution A vial. The initially diluted solution contains 0.8 mg/mL of EPKINLY.
Gently swirl the Dilution A vial for 30 to 45 seconds.
Next, grab the second empty vial labeled “Dilution B”.
Withdraw 2 mL of solution from the Dilution A vial.
Transfer 2 mL of solution from the Dilution A vial into the Dilution B vial.
The Dilution A vial is no longer needed and can be discarded.
Grab your second vial of 0.9% sodium chloride for injection.
Withdraw 8 mL of 0.9% sodium chloride.
Transfer 8 mL of 0.9% Sodium Chloride into the Dilution B vial to make a final concentration of EPKINLY 0.16 mg/mL.
Gently swirl the Dilution B vial for 30 to 45 seconds.
Withdraw 1 mL of the diluted EPKINLY solution from the Dilution B vial into a syringe intended for subcutaneous injection into the patient.
Label the syringe with the dose strength, 0.16 mg, and the time of day.
The syringe is now ready to be administered to the patient.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single-dose vial, meaning multiple doses must not be withdrawn from one vial.
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY.
Chapter 3: 0.8 mg Dose (Step-up Dose 2) EMPTY STERILE VIAL METHOD
Here we will go through the necessary steps needed to prepare the EPKINLY 0.8 mg dose using the empty sterile vial method. Step-up dose 2 is the second dose administered to patients with DLBCL, HGBCL, or FL.
Let's begin by preparing your 0.8 mg dose dilution supplies.
Here is a compilation of all supplies you will need to assemble before you get started.
Now we will go through a simulation on how to prepare a 0.8 mg dose of EPKINLY using empty sterile vials.
Use aseptic technique to prepare EPKINLY.
Filtration of the diluted solution is not required.
To prepare the 0.8 mg dose, EPKINLY 4 mg/0.8 mL requires ONE dilution.
When diluting EPKINLY, use an appropriately sized syringe, vial, and needle for each transfer step.
First, retrieve one 4 mg/0.8 mL EPKINLY vial (with the turquoise cap) from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
Next, grab your appropriately sized empty sterile vial and label the empty vial “Dilution A”.
Grab the 4 mg/0.8 mL EPKINLY vial with the turquoise cap that is now at room temperature.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Withdraw 0.8 mL of EPKINLY using an appropriately sized sterile syringe.
Transfer the 0.8 mL of EPKINLY into the Dilution A vial.
The EPKINLY vial is no longer needed and can be discarded.
REMINDER: Once you have pierced an empty vial, you have equalized the pressure within that vial, so for compounding practices, you need to make sure that you maintain negative pressure within this vial.
Grab your vial of 0.9% Sodium Chloride for injection.
Withdraw 4.2 mL of 0.9% Sodium Chloride.
Transfer 4.2 mL of 0.9% Sodium Chloride into the Dilution A vial to make a final concentration of EPKINLY 0.8 mg/mL.
Gently swirl the Dilution A vial for 30-45 seconds.
Withdraw 1 mL of the diluted EPKINLY solution from the Dilution A vial into a syringe intended for subcutaneous injection in to the patient.
Label the syringe with the dose strength, 0.8 mg, and the time of day.
The syringe is now ready to be administered to the patient.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1. REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit
- Each EPKINLY vial is a single dose vial, meaning multiple doses must not be withdrawn from one vial.
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY.
Chapter 4: 0.16 mg Dose (Step-up Dose 1) STERILE SYRINGE METHOD
We will now review dilution using a sterile syringe method.
Here we will go through the necessary steps needed to prepare the EPKINLY 0.16 mg dose using the sterile syringe method. Step-up dose 1 is the first dose administered to patients with DLBCL, HGBCL, or FL.
Let's begin by preparing your 0.16 mg dose dilution supplies.
Here is a compilation of all sterile supplies you will need to gather before you get started.
Now we will go through a simulation video on how to prepare a 0.16 mg dose of EPKINLY using the sterile syringe method.
Use aseptic technique to prepare EPKINLY. Filtration of the diluted solution is not required.
To prepare the 0.16 mg dose, EPKINLY 4 mg/0.8 mL requires TWO dilutions.
When diluting EPKINLY, use an appropriately sized, syringe, vial, and needle for each transfer step.
First, retrieve one 4 mg/0.8 mL EPKINLY vial (with the turquoise cap) from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
Next, grab your 4 mg/0.8 mL EPKINLY vial with the turquoise cap that is now at room temperature.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Retrieve four appropriately sized syringes. Label one “Dilution A” and another “Dilution B”. Then, label another syringe “Syringe 1” and the last one “Syringe 2”.
Withdraw 0.8 mL of EPKINLY into Syringe 1.
Grab one vial of 0.9% Sodium Chloride for injection.
Withdraw 4.2 mL of 0.9% Sodium Chloride into the Dilution A syringe. Include approximately 0.2 mL of air in the syringe.
Connect Syringe 1 containing 0.8 mL of EPKINLY with the Dilution A syringe containing 0.9% Sodium Chloride. Push the 0.8 mL of EPKINLY into the Dilution A syringe. The initially diluted solution contains 0.8 mg/mL of EPKINLY.
Gently mix by inverting the connected syringes 180 degrees 5 times.
Disconnect the syringes and discard Syringe 1.
Connect Syringe 2 to the Dilution A syringe and transfer 2 mL of solution into Syringe 2.
The Dilution A syringe is no longer needed.
Next, withdraw 8 mL of 0.9% Sodium Chloride into the Dilution B syringe. Include approximately 0.2 mL of air in the syringe.
Connect Syringe 2 to the Dilution B syringe. Push the 2 mL of EPKINLY solution into the Dilution B syringe to make a final concentration of 0.16 mg/mL.
Gently mix by inverting the connected syringes 180 degrees 5 times.
Disconnect the syringes and discard Syringe 2.
Connect the Dilution B syringe to a new syringe intended for administration to the patient.
Withdraw 1 mL of the diluted EPKINLY solution from the Dilution B syringe into the new syringe intended for administration to the patient.
The Dilution B syringe is no longer needed.
Label the syringe with the dose strength, 0.16 mg, and the time of day.
The syringe is now ready to be administered to the patient via subcutaneous injection.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY.
Chapter 5: 0.8 mg Dose (Step-up Dose 2) STERILE SYRINGE METHOD
Here we will go through the necessary steps needed to prepare the EPKINLY 0.8 mg dose using the sterile syringe method. Step-up dose 2 is the second dose administered to patients with DLBCL, HGBCL, or FL.
Let's begin by preparing your 0.8 mg dose dilution supplies.
Here is a compilation of all sterile supplies you will need to gather before you get started.
Now we will go through a simulation video on how to prepare a 0.8 mg dose of EPKINLY using the sterile syringe method.
Use aseptic technique to prepare EPKINLY. Filtration of the diluted solution is not required.
To prepare the 0.8 mg dose, EPKINLY 4 mg/0.8 mL requires ONE dilution.
When diluting EPKINLY, use an appropriately sized syringe and needle for each transfer step.
First, retrieve one 4 mg/0.8 mL EPKINLY vial (with the turquoise cap) from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
Retrieve two appropriately sized syringes. Label one “Dilution A”, and another “Syringe 1”.
Grab the 4 mg/0.8 mL EPKINLY vial with the turquoise cap that is now at room temperature.
Gently swirl the EPKINLY vial. Do not invert, vortex, or vigorously shake the vial.
Withdraw 0.8 mL of EPKINLY into Syringe 1.
Grab one vial of 0.9% Sodium Chloride for injection.
Withdraw 4.2 mL of 0.9% Sodium Chloride into the Dilution A syringe. Include approximately 0.2 mL of air in the syringe.
Connect Syringe 1 containing 0.8 mL of EPKINLY with the Dilution A syringe containing 0.9% Sodium Chloride. Push the 0.8 mL of EPKINLY into the Dilution A syringe to make a final concentration of
0.8 mg/mL.
Gently mix by inverting the connected syringes 180 degrees 5 times.
Disconnect the syringes and discard Syringe 1.
Connect the syringe marked Dilution A to a new syringe intended for administration to the patient.
Withdraw 1 mL of the diluted EPKINLY solution from the Dilution A syringe into the new syringe intended for administration to the patient.
The Dilution A syringe is no longer needed.
Label the syringe with the dose strength, 0.8 mg, and the time of day.
The syringe is now ready to be administered to the patient via subcutaneous injection.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single dose vial and any unused portion of EPKINLY.
Chapter 6: 3 mg Dose (Step-up Dose 3) FL ONLY
Now we will go through a simulation video on how to prepare a 3 mg dose of EPKINLY. EPKINLY 3 mg step-up dose is required for FL patients only.
Please note that the 3 mg dose is prepared from a ready-to-use solution and does not require dilution.
Let's begin by preparing your 3 mg dose supplies.
Here is a compilation of all supplies you will need to assemble before you get started.
Here we will go through a simulation on how to prepare a 3 mg dose of EPKINLY.
Use aseptic technique to prepare EPKINLY.
First, retrieve one 4 mg/0.8 mL EPKINLY vial with the turquoise cap from the refrigerator.
Allow the vial to come to room temperature for no more than 1 hour.
Gently swirl the EPKINLY vial.
Do not invert, vortex, or vigorously shake the vial.
Withdraw 0.6 mL of EPKINLY into a syringe intended for subcutaneous injection into the patient.
Label the syringe with the dose strength, 3 mg, and the time of day.
The syringe is now ready to be administered to the patient.
Refer to Storage & Handling instructions for EPKINLY solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single-dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY.
Chapter 7: 48 mg Dose (Full Dose)
Lastly, we will go through the necessary steps needed to prepare the EPKINLY 48 mg dose. This is the full dose administered to patients with DLBCL, HGBCL, or FL.
Please note that the 48 mg dose is prepared from a ready-to-use solution and does not require dilution.
Let's begin by preparing your 48 mg dose supplies.
Here is a compilation of all supplies you will need to assemble before you get started.
Here we will go through a simulation on how to prepare a 48 mg dose of EPKINLY.
Use aseptic technique to prepare EPKINLY.
First, retrieve the 48 mg/0.8 mL EPKINLY vial with the orange cap from the refrigerator.
Allow the vial to come to room temperature for no more than one hour.
Gently swirl the EPKINLY vial.
DO NOT invert, vortex, or vigorously shake the vial.
Withdraw 0.8 mL of EPKINLY into a syringe intended for a subcutaneous injection into the patient.
Label the syringe with the dose strength, 48 mg, and the time of day.
The syringe is now ready to be administered to the patient.
Refer to Storage & Handling instructions for EPKINLY Solution in the syringe in Chapter 1.
REMINDER:
- EPKINLY is for subcutaneous injection ONLY
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit
- Each EPKINLY vial is a single dose vial, meaning multiple doses must not be withdrawn from one vial
- After preparing each dose, discard the single-dose vial and any unused portion of EPKINLY
Chapter 8: Important Safety Information for EPKINLY® (epcoritamab-bysp)
The following is the important safety information for EPKINLY.
BOXED WARNINGS
- Cytokine release syndrome (CRS), including serious or fatal reactions, can occur in patients receiving EPKINLY. Initiate treatment with the EPKINLY step-up dosage schedule to reduce the incidence and severity of CRS. Withhold EPKINLY until CRS resolves or permanently discontinue based on severity.
- Immune effector cell–associated neurotoxicity syndrome (ICANS), including life-threatening and fatal reactions, can occur with EPKINLY. Monitor patients for neurological signs or symptoms of ICANS during treatment. Withhold EPKINLY until ICANS resolves or permanently discontinue based on severity.
CRS
- CRS occurred in 51% (80/157) of patients with large B-cell lymphoma (LBCL) in the clinical trial (37% Grade 1, 17% Grade 2, and 2.5% Grade 3) and recurred in 31% of patients. Most events (92%) occurred during Cycle 1. In Cycle 1, CRS events occurred in 6% of patients after the 0.16 mg dose (Cycle 1, day 1), 12% after the 0.8 mg dose (Cycle 1, day 8), 43% after the first 48 mg dose (Cycle 1, day 15), and 5% after the next 48 mg dose (Cycle 1, day 22). The median time to onset of CRS from the most recent administered dose across all doses was 24 hours (range: 0-10 days). The median time to onset after the first full 48 mg dose was 21 hours (range: 0-7 days).
- Patients with DLBCL or high-grade B-cell lymphoma should be hospitalized for 24 hours following administration of the first full 48 mg dose.
- CRS occurred in 49% (42/86) of patients with FL receiving the recommended 3-step up dosage schedule in the clinical trial (45% Grade 1, 9% Grade 2) and recurred in 48% of patients. Most events (88%) occurred during Cycle 1. In Cycle 1, CRS events occurred in 12% of patients after the 0.16 mg dose (Cycle 1, day 1), 6% after the 0.8 mg dose (Cycle 1, day 8), 15% after the 3 mg dose (Cycle 1, day 15), and 37% after the first 48 mg dose (Cycle 1, day 22). The median time to onset of CRS from the most recent administered dose across all doses was 59 hours (range: 0.1-7 days). The median time to onset after the first full 48 mg dose was 61 hours (range: 0.1-7 days).
- CRS occurred in 24% (32/131) of patients with FL receiving EPKINLY at the recommended dosage schedule in combination with lenalidomide and rituximab in the clinical trial (19% Grade 1, 5% Grade 2, and 12% serious adverse reactions due to CRS) and recurred in 41% of patients. Most events (88%) occurred during Cycle 1. In Cycle 1, CRS occurred in 5% of patients after the 0.16 mg dose (Cycle 1, day 1), 3.8% after the 0.8 mg dose (Cycle 1, day 8), 2.3% after the 3 mg dose (Cycle 1, day 15), and 18% after the first 48 mg dose (Cycle 1, day 22). The median time to onset of CRS from the most recent EPKINLY dose was 78 hours (range: 0.2 to 12 days). The median time to onset after the first 48 mg dose was 41 hours (range 0.3-12 days).
- For patients with FL, assess whether hospitalization or outpatient monitoring for the first 48 mg dose is appropriate based on comorbidities or other situational factors. During outpatient monitoring after the first 48 mg dose, patients should remain in proximity to a healthcare facility that can assess and manage CRS.
- In patients who experienced CRS, the signs and symptoms included pyrexia, hypotension, hypoxia, dyspnea, chills, and tachycardia. CRS resolved in 98% of patients; the median duration of CRS events was 2 days (range: <1-27 days). Concurrent neurological adverse reactions associated with CRS occurred in 2.5% of patients with LBCL, 4.7% of patients with FL receiving EPKINLY monotherapy, and 1.5% of patients receiving EPKINLY in combination with lenalidomide and rituximab (reactions included headache, confusional state, tremors, dizziness, and ataxia).
- Administer pretreatment medications to reduce the risk of CRS
- Monitor patients for potential CRS. At the first signs or symptoms of CRS, immediately evaluate patients for hospitalization, manage per current practice guidelines, and administer supportive care as appropriate.
ICANS
- ICANS occurred in 6% (10/157) of patients with LBCL in the clinical trial (4.5% Grade 1, 1.3% Grade 2, 0.6% fatal). Of the 10 ICANS events, 9 occurred within Cycle 1 of treatment, with a median time to onset of 16.5 days (range: 8-141 days) from the start of treatment. Relative to the most recent administered dose, the median time to onset was 3 days (range: 1-13 days). The median duration of ICANS was 4 days (range: 0-8 days), with ICANS resolving in 90% of patients with supportive care.
- ICANS occurred in 6% (8/127) of patients with FL receiving the 2-step up dosage schedule in the clinical trial (3.9% Grade 1, 2.4% Grade 2). The median time to onset was 22 days (range: 14-66 days) from the start of treatment. Relative to the most recent administered dose, the median time to onset of ICANS was 3 days (range: 0.4-7 days). The median duration of ICANS was 2 days (range: 1-7 days), with ICANS resolving in 100% of patients.
- Among patients with FL who received EPKINLY at the recommended dosage schedule in combination with lenalidomide and rituximab in the clinical trial, ICANS occurred in 0.8% (1/131, Grade 1).
- The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. Clinical manifestations of ICANS included, but were not limited to, confusional state, lethargy, tremor, dysgraphia, aphasia, and non-convulsive status epilepticus.
- Monitor patients for potential ICANS. At the first signs or symptoms of ICANS, immediately evaluate patient, provide supportive therapy based on severity, and manage per current practice guidelines.
Infections
- EPKINLY can cause fatal and serious infections. Serious infections, including opportunistic infections, were reported in 15% of patients with LBCL in the clinical trial (most common: 4.5% sepsis, 3.2% pneumonia). Fatal infections occurred in 1.3% of patients (1.3% COVID-19).
- Serious infections, including opportunistic infections, were reported in 40% of patients with FL receiving EPKINLY monotherapy, following the 2-step up dosage schedule in the clinical trial (most common: 20% COVID-19, 13% pneumonia, 3% urinary tract infections). Fatal infections occurred in 6% of patients (5% COVID-19, 0.8% pneumonia, 0.8% sepsis).
- Among 243 patients with FL who received EPKINLY in combination with lenalidomide and rituximab in the clinical trial, serious infections occurred in 28% of patients. The most common serious infections were pneumonia (15%), COVID-19 (7%), opportunistic infections (5%) and upper respiratory infections (3.3%). The most common opportunistic infections of any grade were CMV (cytomegalovirus) infection (7%) and herpesvirus infection (7%).
- Progressive multifocal leukoencephalopathy (PML), including fatal cases, has occurred in patients treated with EPKINLY. Across a broader clinical trial population, PML was reported in 0.4% (11/3072) of patients, including in the first-line treatment setting. Of the 11 cases of PML, 6 resulted in fatal outcomes and 1 was unresolved at the time of death.
- Monitor patients for signs and symptoms of infection and treat appropriately. Avoid administration in patients with active infections. Withhold or consider permanent discontinuation of EPKINLY based on severity. Provide Pneumocystis jirovecii pneumonia (PJP) prophylaxis during treatment with EPKINLY, and consider prophylaxis against herpesvirus.
Cytopenias
- EPKINLY can cause serious or severe cytopenias. In the clinical trial of patients with LBCL, Grade 3 or 4 decreased neutrophils occurred in 32% (Grade 4, 14%), decreased hemoglobin in 12% (Grade 4, 0%), and decreased platelets in 12% (Grade 4, 7%) of patients. Febrile neutropenia occurred in 2.5% (Grade 4, 0.6%).
- In the clinical trial of patients with FL who received EPKINLY monotherapy following the 2-step up dosage schedule, Grade 3 or 4 decreased neutrophils occurred in 30% (Grade 4, 17%), decreased hemoglobin in 10% (Grade 4, 0%), and decreased platelets in 8% (Grade 4, 4%) of patients. Febrile neutropenia occurred in 3.1% (Grade 4, 0%).
- In patients with FL who received EPKINLY in combination with lenalidomide and rituximab, Grade 3 or 4 decreased neutrophils occurred in 67% (Grade 4, 41%), decreased lymphocytes in 62% (Grade 4, 13%), decreased hemoglobin in 7%, and decreased platelets in 10% (Grade 4, 4.1%) of patients. Febrile neutropenia occurred in 6% (Grade 4, 2.1%).
- Monitor complete blood counts throughout treatment. Based on severity of cytopenias, temporarily withhold or permanently discontinue EPKINLY. Consider prophylactic granulocyte colony-stimulating factor administration as applicable.
Embryo-Fetal Toxicity
- EPKINLY may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with EPKINLY and for 4 months after the last dose. Verify pregnancy status in females of reproductive potential prior to initiating EPKINLY.
Adverse Reactions
- EPKINLY as monotherapy for LBCL or FL: Most common (≥20%) adverse reactions were CRS, injection site reactions, fatigue, musculoskeletal pain, fever, diarrhea, COVID-19, rash, and abdominal pain. Most common Grade 3 to 4 laboratory abnormalities (≥10%) were decreased lymphocytes, decreased neutrophils, decreased hemoglobin, and decreased platelets.
- EPKINLY in combination with lenalidomide and rituximab for FL: Most common (≥20%) adverse reactions were rash, upper respiratory tract infections, fatigue, injection site reactions, constipation, diarrhea, CRS, pneumonia, COVID-19, and fever. The most common Grade 3 to 4 laboratory abnormalities (≥10%) were decreased neutrophils, decreased lymphocytes, and decreased platelets.
Use in Specific Populations
- Lactation: Advise women not to breastfeed during treatment and for 4 months after the last dose of EPKINLY.
- Geriatric Use: In patients with relapsed or refractory FL who received EPKINLY in the clinical trial, 52% were ≥65 years old, and 13% were ≥75 years old. A higher rate of fatal adverse reactions, primarily infections, including COVID-19, was observed in patients ≥65 years old compared to younger adult patients. No overall difference in efficacy was observed.
Please see link above for Full Prescribing Information.
3L=third line; CRS=cytokine release syndrome; ICANS=immune effector cell-associated neurotoxicity syndrome; IV=intravenous; mg=milligram; mL=milliliter; W1=week 1; W2=week 2; W3=week 3; W4=week 4.



