3L+ DLBCL Treatment Landscape
Patients need options in 3L+ DLBCL that are efficacious, tolerable, and accessible1
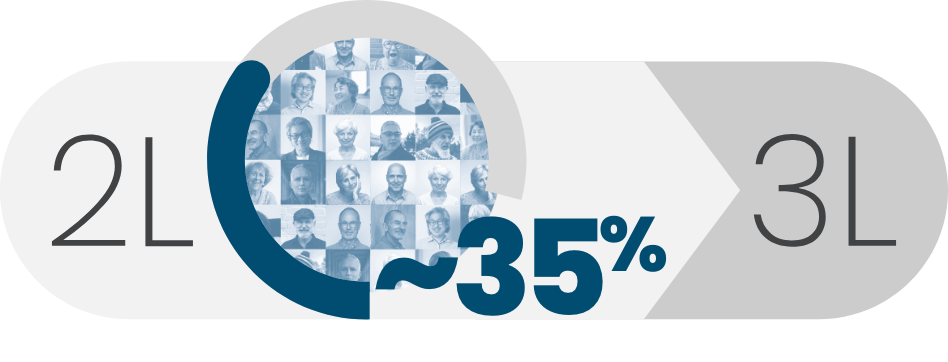
of DLBCL patients who relapsed or were refractory to 2L received 3L treatment2,3
The treatment landscape is rapidly evolving, and statistics may not reflect current outcomes.
By 3L+, patients have a poor prognosis, facing low response and worsening survival rates with each subsequent line of therapy4
- Patients who relapse post-2L ASCT have rapid disease progression4
- Patients who relapse post-3L CAR T experience poor outcomes5-7
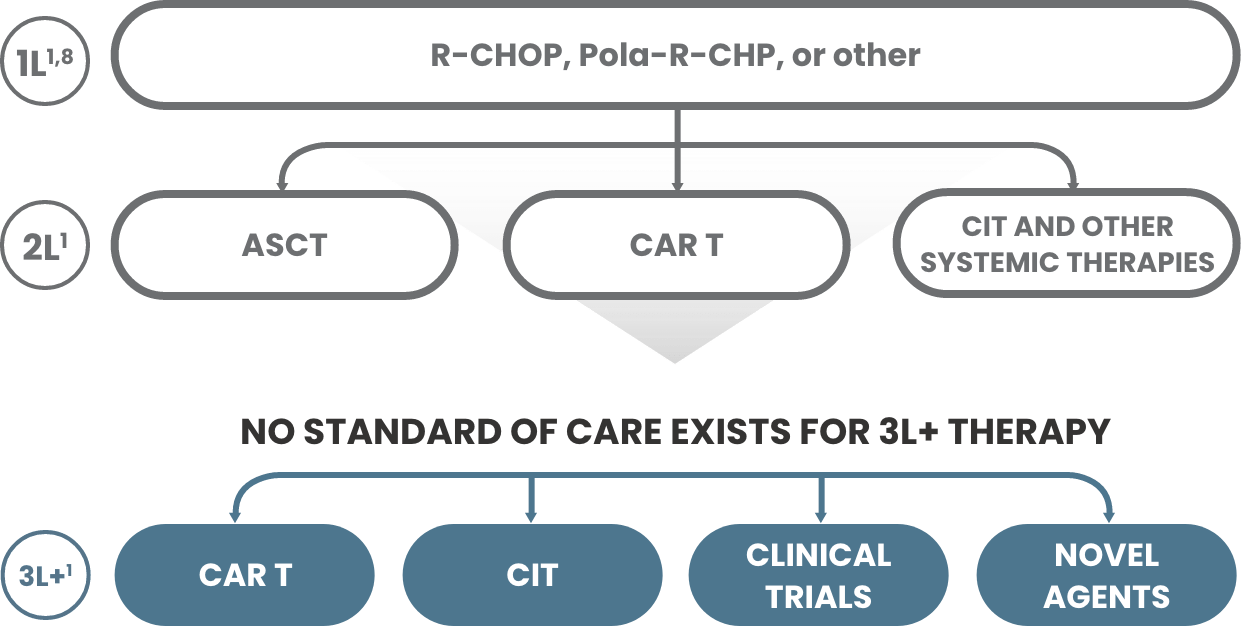
The currently approved 3L+ DLBCL treatments may not be suitable for all patients due to both clinical and nonclinical factors, including1,9-11:
- Comorbidities
- Logistics or manufacturing
- Insufficient care partner support
- Total cost of care to patient
While some patients with 3L+ DLBCL may achieve remission, the approved options are not always accessible or effective for all patients12,13
1L=first line; 2L=second line; 3L=third line; ASCT=autologous stem cell transplant; CAR T=chimeric antigen receptor T‑cell; CIT=chemoimmunotherapy; DLBCL=diffuse large B‑cell lymphoma; Pola-R-CHP=polatuzumab vedotin + rituximab, cyclophosphamide, doxorubicin, prednisone; R-CHOP=rituximab + cyclophosphamide, doxorubicin, vincristine, prednisone.


