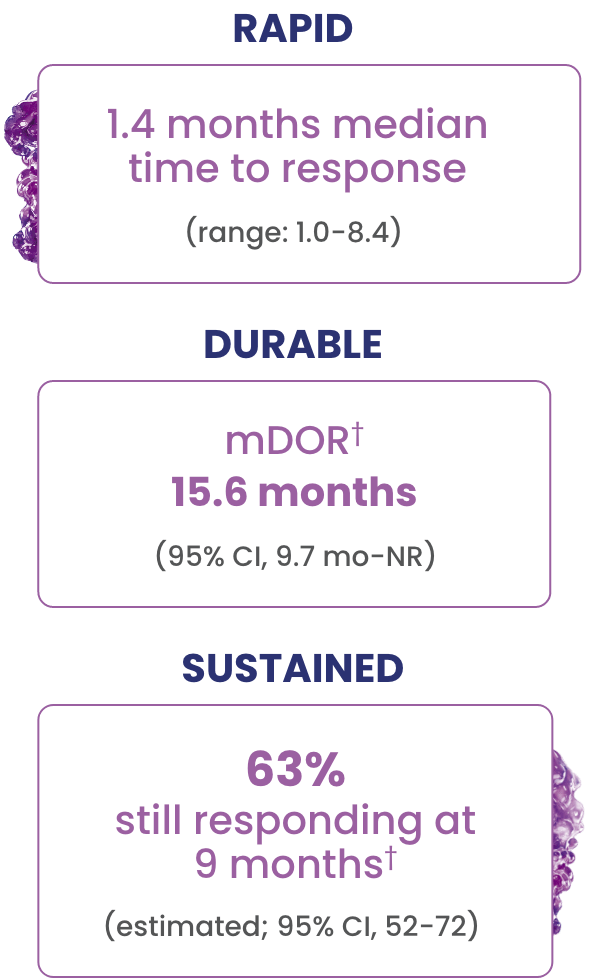
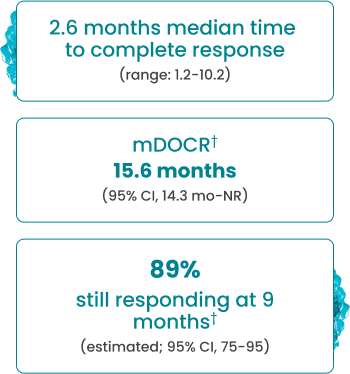
EPKINLY delivered an ORR of 61%, with 38% of patients achieving a deep response of CR1
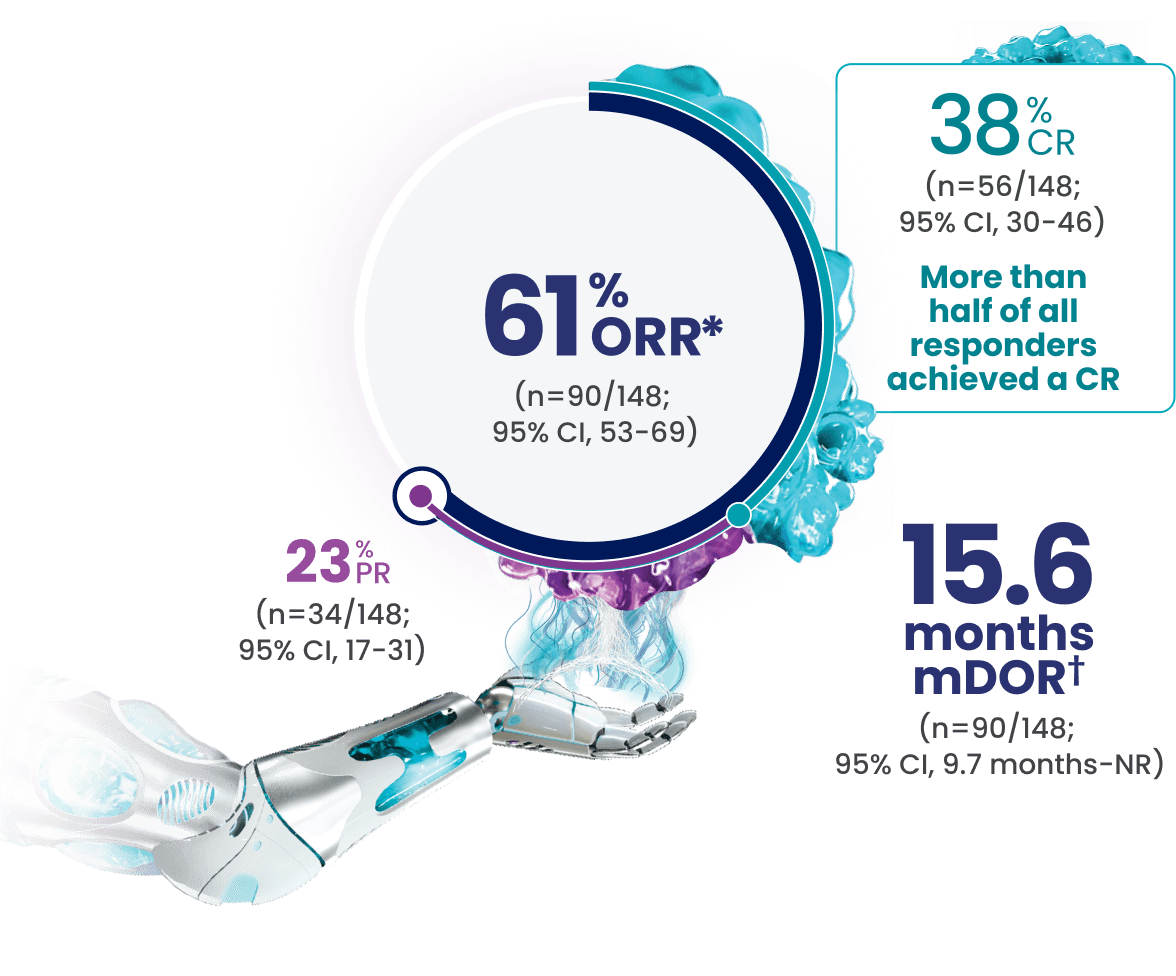
*Efficacy results determined by Lugano criteria (2014) as assessed by Independent Review Committee (IRC).
†Based on Kaplan-Meier estimate.
ORR, CR, and PR rates observed across additional subgroups1,2*
The efficacy of EPKINLY was evaluated in EPCORE NHL-1, an open-label, multicohort, multicenter, single-arm trial in 148 patients with R/R DLBCL after 2 or more lines of systemic therapy.

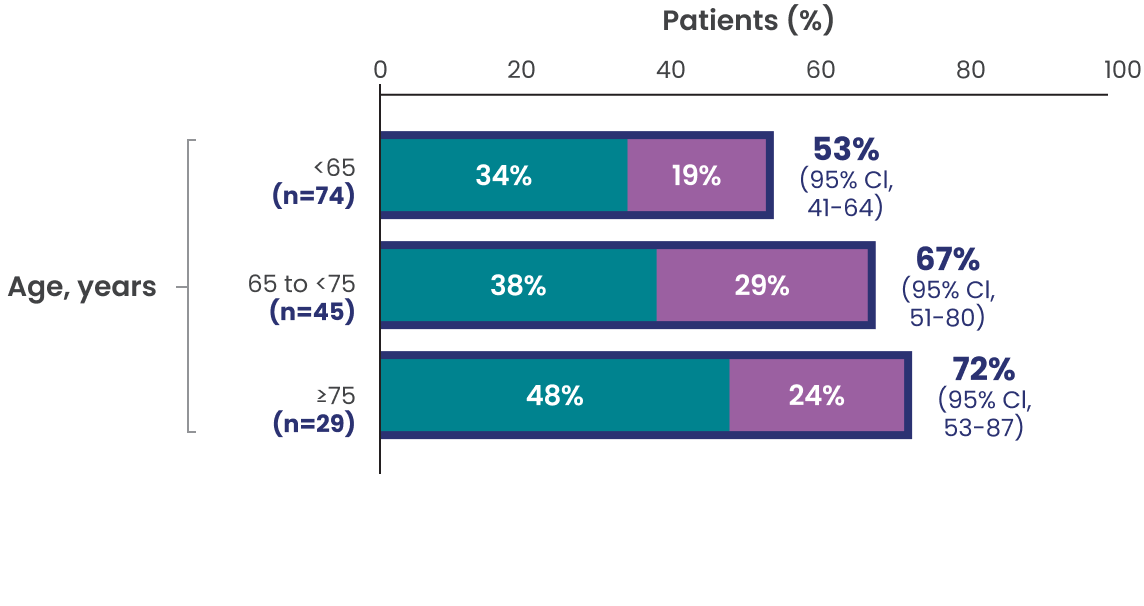
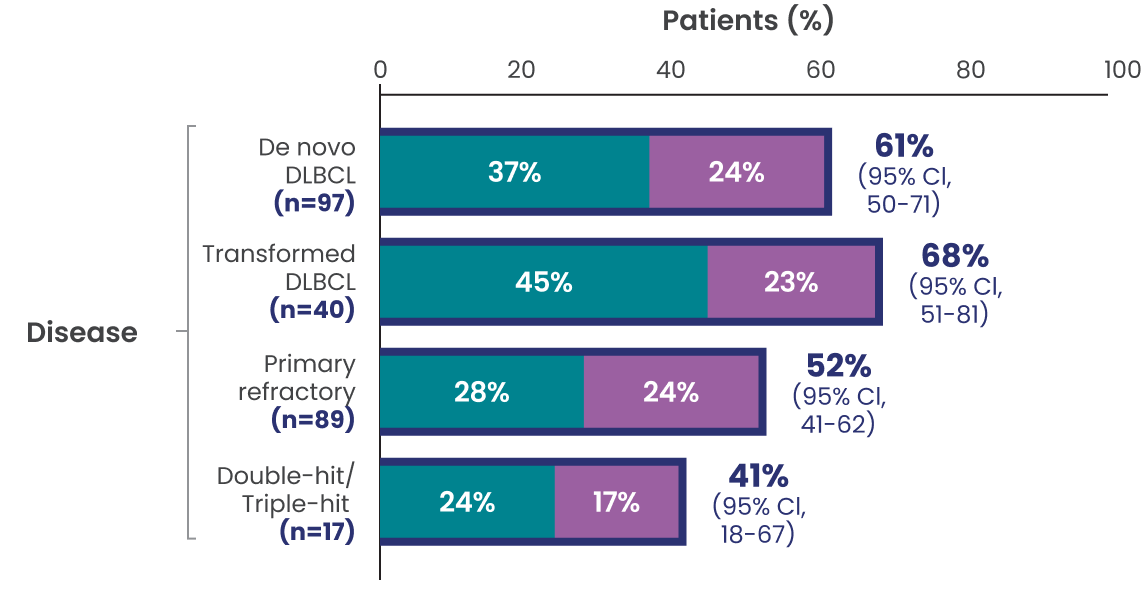
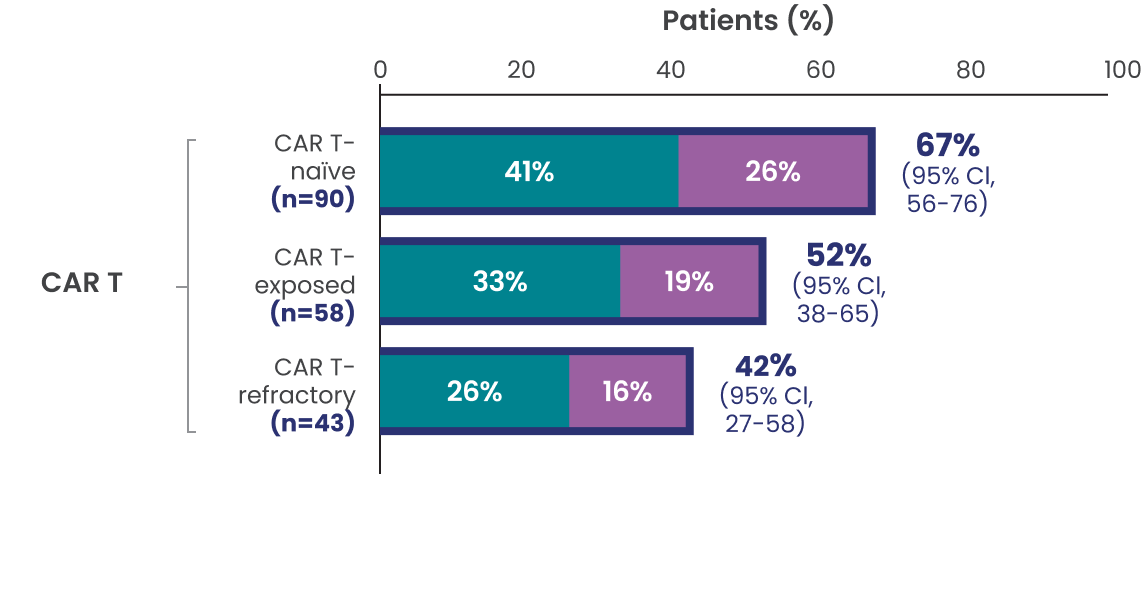
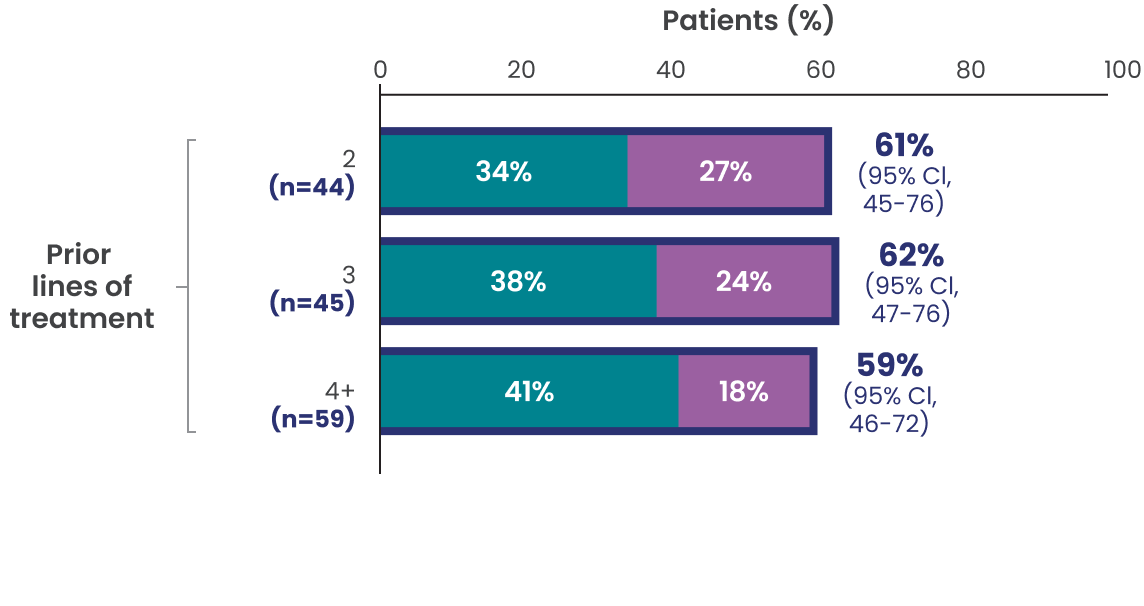
Data Limitation: Study was not powered to evaluate these prespecified subgroups. Data are exploratory and descriptive in nature. No formal inferences can be drawn.
*Efficacy results determined by Lugano criteria (2014) as assessed by Independent Review Committee (IRC).
In overall responders (61%, n=90/148), EPKINLY delivered durable responses in heavily pretreated 3L+ DLBCL, NOS patients1
- Complete responses were achieved as late as 10.2 months2
- The median follow-up for DOCR was 9.7 months (range: 8.3-12.1 months)2
†Based on Kaplan-Meier estimate.
Long-term follow-up data3
Investigator-assessed DOR and DOCR at a median study follow-up of 3 years*
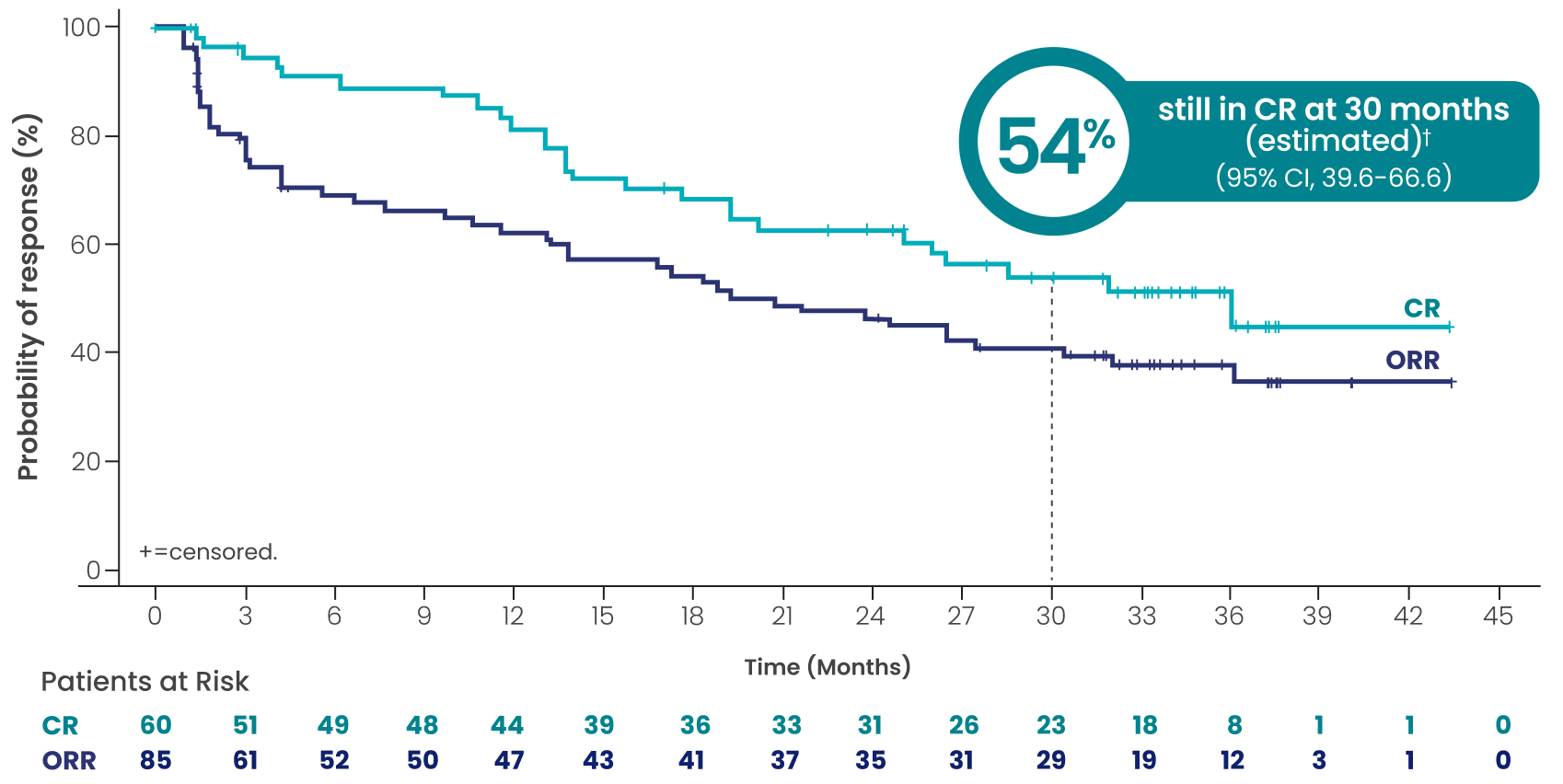
ORR=57.4% (n=85/148; 95% CI, 49.0-65.5)
CR=40.5% (n=60/148; 95% CI, 32.6-48.9)
PR=16.9% (n=25/148; 95% CI, 11.2-23.9)
mDOCR†‡ 36.1 months
(95% CI, 20.2-NR)
mDOR†‡ 20.8 months
(95% CI, 13.0-32.0)
Overall median study follow-up was 37.2 months (range: 0.3+, 45.5).§
Efficacy results determined by Lugano criteria per investigator assessment (INV).
Data cutoff: May 3, 2024.
Data Limitations:
No inference can be drawn from this data set. Follow-up analysis is exploratory and data are descriptive in nature.
The Kaplan-Meier estimates may be unreliable at the tail end of the curve due to a smaller number of patients at risk.
Long-term follow-up data across subgroups4
Select a tab to explore more content
CAR-T–exposed (n=58): Investigator-assessed DOR and
DOCR at a median study follow-up of 3 years
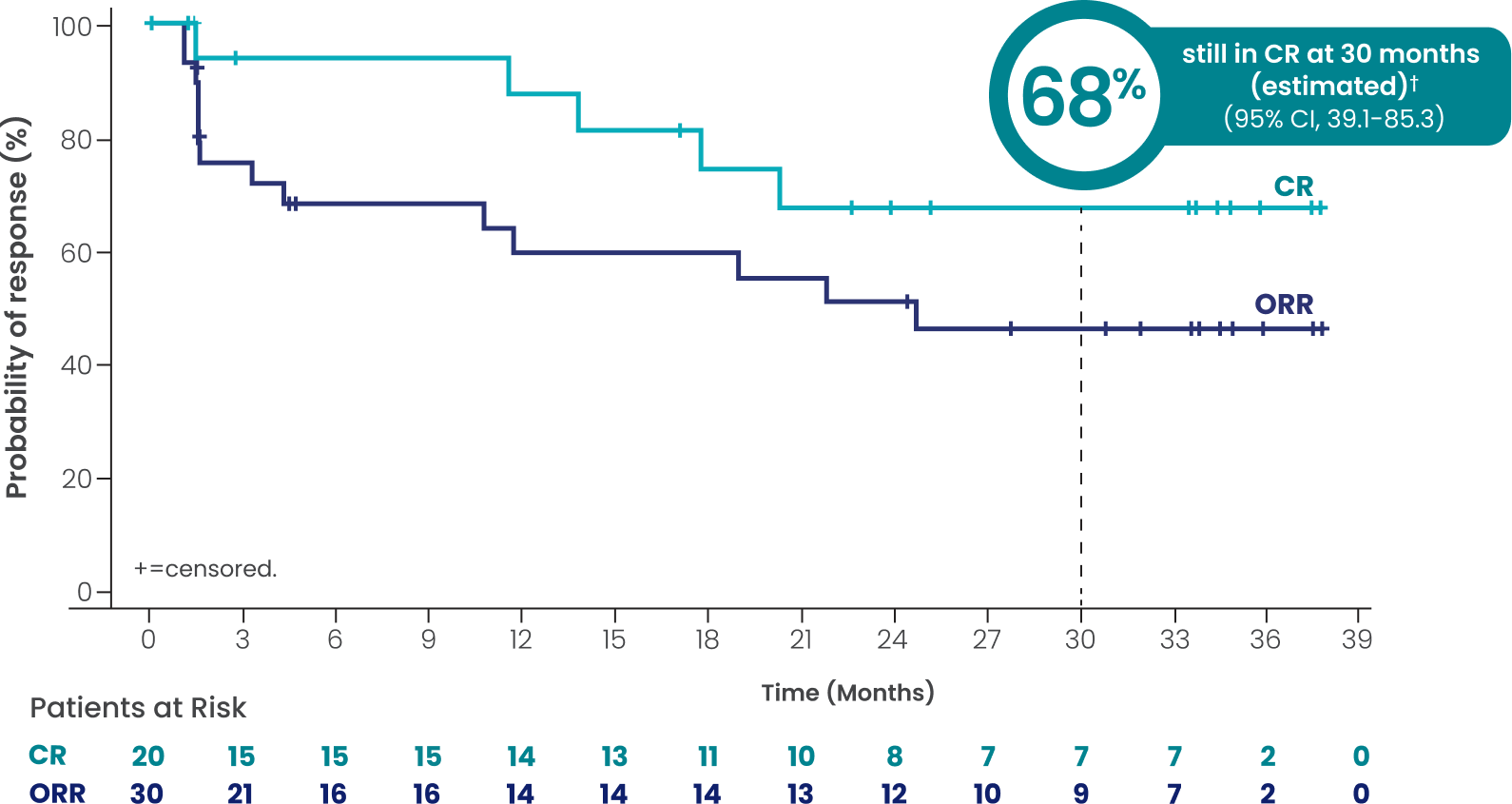
Overall median study follow-up was 37.2 months (range: 0.3+, 45.5).§
Efficacy results determined by Lugano criteria per investigator assessment (INV).
Data cutoff: May 3, 2024.
Data Limitations:
Study was not powered to evaluate these prespecified subgroups; data are exploratory and descriptive in nature and no formal inferences can be drawn.
The Kaplan-Meier estimates may be unreliable at the tail end of the curve due to a smaller number of patients at risk.
ORR=51.7% (n=30/58; 95% CI, 38.2-65.0)
CR=34.5% (n=20/58; 95% CI, 22.5-48.1)
PR=17.2% (n=10/58; 95% CI, 8.6-29.4)
mDOCR†∥ not reached
(95% CI, 17.7-NR)
mDOR†∥ 24.6 months
(95% CI, 4.2-NR)
Data Limitations:
Study was not powered to evaluate these prespecified subgroups; data are exploratory and descriptive in nature and no formal inferences can be drawn.
The Kaplan-Meier estimates may be unreliable at the tail end of the curve due to a smaller number of patients at risk.
CAR-T–refractory (n=43): Investigator-assessed DOR
and DOCR at a median study follow-up of 3 years
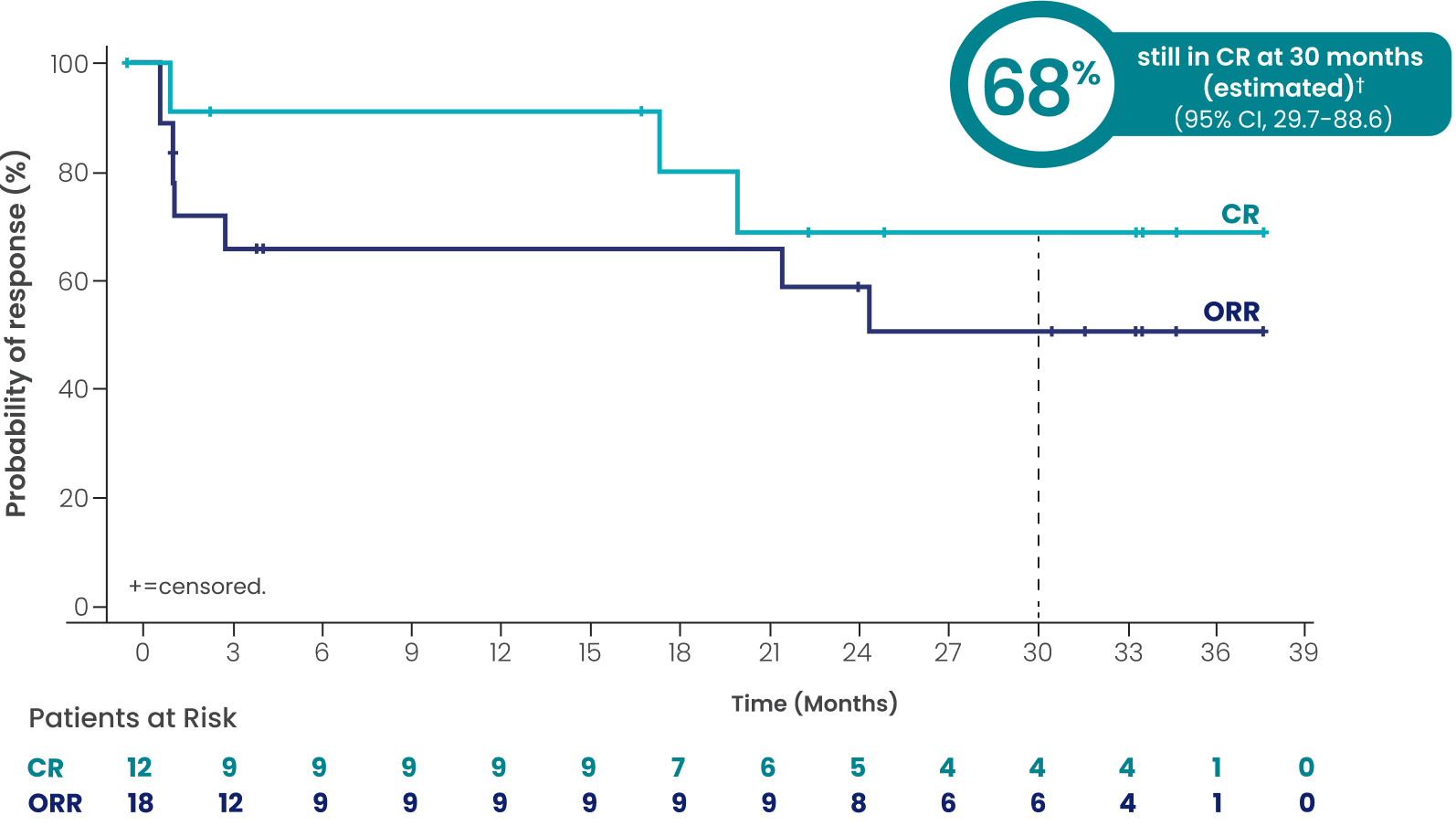
Overall median study follow-up was 37.2 months (range: 0.3+, 45.5).§
Efficacy results determined by Lugano criteria per investigator assessment (INV).
Data cutoff: May 3, 2024.
Data Limitations:
Study was not powered to evaluate these prespecified subgroups; data are exploratory and descriptive in nature and no formal inferences can be drawn.
The Kaplan-Meier estimates may be unreliable at the tail end of the curve due to a smaller number of patients at risk.
ORR=41.9% (n=18/43; 95% CI, 27.0-57.9)
CR=27.9% (n=12/43; 95% CI, 15.3-43.7)
PR=14.0% (n=6/43; 95% CI, 5.3-27.9)
mDOCR†¶ not reached
(95% CI, 17.7-NR)
mDOR†¶ 24.6 months
(95% CI, 1.4-NR)
Data Limitations:
Study was not powered to evaluate these prespecified subgroups; data are exploratory and descriptive in nature and no formal inferences can be drawn.
The Kaplan-Meier estimates may be unreliable at the tail end of the curve due to a smaller number of patients at risk.
Transformed disease from FL (n=32): Investigator-assessed DOR and DOCR
at a median study follow-up of 3 years
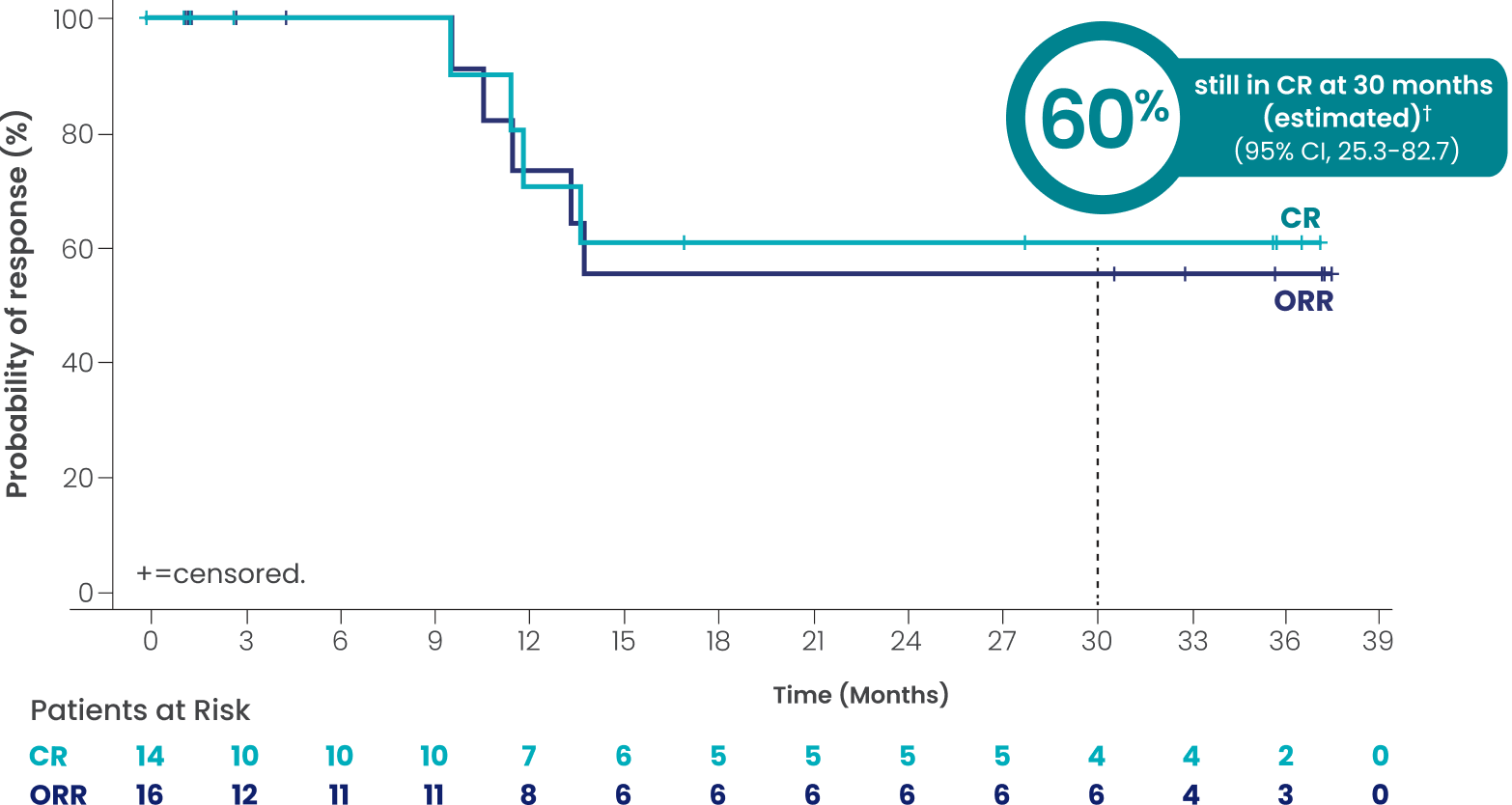
Overall median study follow-up was 37.2 months (range: 0.3+, 45.5).§
Efficacy results determined by Lugano criteria per investigator assessment (INV).
Data cutoff: May 3, 2024.
Data Limitations:
Study was not powered to evaluate these prespecified subgroups; data are exploratory and descriptive in nature and no formal inferences can be drawn.
The Kaplan-Meier estimates may be unreliable at the tail end of the curve due to a smaller number of patients at risk.
ORR=50.0% (n=16/32; 95% CI, 31.9-68.1)
CR=43.8% (n=14/32; 95% CI, 26.4-62.3)
PR=6.3% (n=2/32; 95% CI, 0.8-20.8)
mDOCR†# not reached
(95% CI, 9.7-NR)
mDOR†# not reached
(95% CI, 10.6-NR)
Data Limitations:
Study was not powered to evaluate these prespecified subgroups; data are exploratory and descriptive in nature and no formal inferences can be drawn.
The Kaplan-Meier estimates may be unreliable at the tail end of the curve due to a smaller number of patients at risk.
Long-term follow-up: Safety data3
- With a median follow-up of 3 years, observations were consistent with the known epcoritamab safety profile. Discontinuation due to an adverse reaction occurred in 7.6% of patients
- Serious infections were reported in 31% of patients. Serious infections ≥5% were COVID-19 events** (17% of patients) and pneumonia (5% of patients). Fatal infections occurred in 14 patients, of which 10 were COVID-19 events**
*Efficacy results determined by Lugano criteria (2014) as assessed by Independent Review Committee (IRC).1
†Based on Kaplan-Meier estimate.4
‡Median follow-up for DOR was 33.6 months (range: 32.7-37.2 months). Median follow-up for DOCR was 33.4 months (range: 31.8-34.9 months).§
§Based on reverse Kaplan-Meier estimate.4
||Median follow-up for DOR was 33.4 months (95% CI, 24.2-34.8 months). Median follow-up for DOCR was 33.4 months (95% CI, 17.1-34.8 months).4§
¶Median follow-up for DOR was 31.7 months (95% CI: 4.4-34.8 months). Median follow-up for DOCR was 25.1 months (95% CI, 2.8-34.8 months).4§
#Median follow-up for DOR was 32.9 months (95% CI, 2.8-37.3 months). Median follow-up for DOCR was 27.9 months (95% CI, 1.4-36.6 months).4§
**COVID-19 events represent COVID-19 and COVID-19 pneumonia.3
3L=third line; CAR T=chimeric antigen receptor T-cell therapy; CI=confidence interval; CR=complete response; DOCR=duration of complete response; DOR=duration of response; DLBCL=diffuse large B-cell lymphoma; FL=follicular lymphoma; HSCT=hematopoietic stem cell transplant; mDOCR=median duration of complete response; mDOR=median duration of response; NOS=not otherwise specified; NR=not reached; ORR=overall response rate; PR=partial response.

Find out more about clinical trial treatment-related adverse reactions that occurred with EPKINLY