
The power of superior PFS with fixed-duration EPKINLY + R2 vs R2 1*†
EPKINLY + R2 demonstrated a 79% reduction in the risk of disease progression or death vs R2 (HR=0.21‡; 95% CI, 0.13-0.33; P<0.0001§)
Date of analysis: January 10, 2025.²
*Efficacy results determined by Lugano criteria (2014) as assessed by IRC and based on prespecified interim analysis.
†The median duration of study follow-up was 10.4 months in the ITT population.
‡Cox proportional hazards hazard ratio stratified by disease history and region.
§Log-rank P-value (one-sided) stratified by disease history and region.
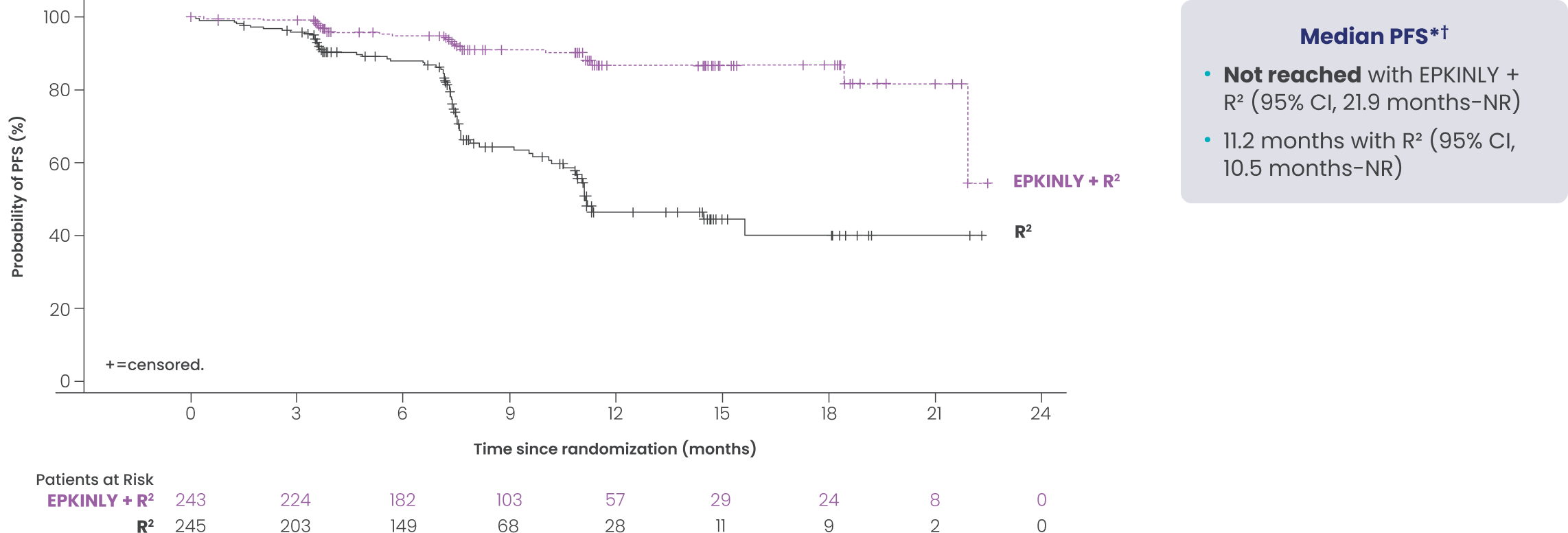
EPKINLY + R2 PFS benefit was consistent between the pivotal and follow-up analyses1,2
EPCORE® FL-1 prespecified follow-up analysis PFS results*†
Data Limitation: These prespecified efficacy analyses are not multiplicity-controlled. Data are exploratory and descriptive in nature. No formal inferences can be drawn.
Date of analysis: May 24, 2025.
The median duration of study follow-up was 14.8 months.
*Efficacy results determined by Lugano criteria as assessed by IRC and based on a prespecified interim analysis.
†Based on Kaplan-Meier analysis.
‡Stratified by disease history/status. Hazard ratio is estimated using Cox proportional hazards model.
PFS observed across subgroups2
EPCORE® FL-1 prespecified follow-up analysis PFS results
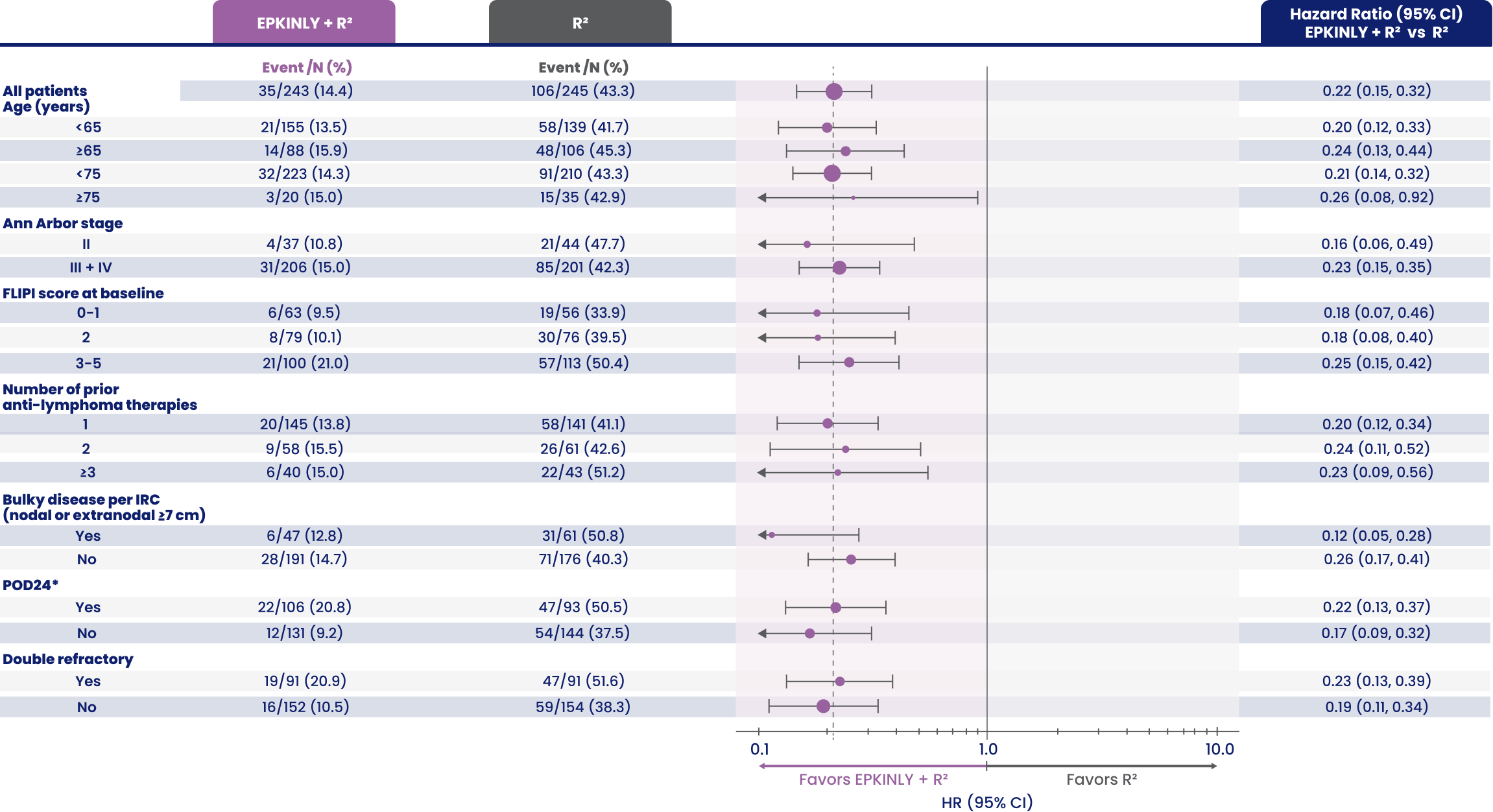
Arrows indicate that the confidence interval is extended more than current range.
Data Limitation: These prespecified analyses are not multiplicity-controlled. Data are exploratory and descriptive in nature. No formal inferences can be drawn.
*POD24 is defined as progression of disease ≤2 years from the date of initiation of first-line chemoimmunotherapy.
EPKINLY + R2 achieved remarkable responses with a 31% higher CR vs R2 1*
EPCORE® FL-1 pivotal analysis
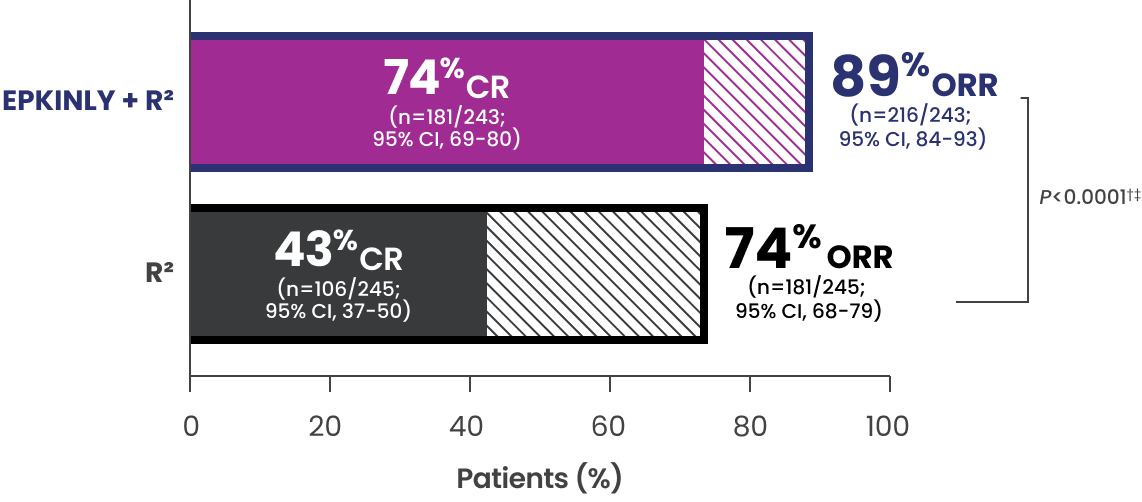
- The median duration of follow-up was 10.4 months in the ITT population
EPCORE® FL-1 prespecified follow-up analysis2§
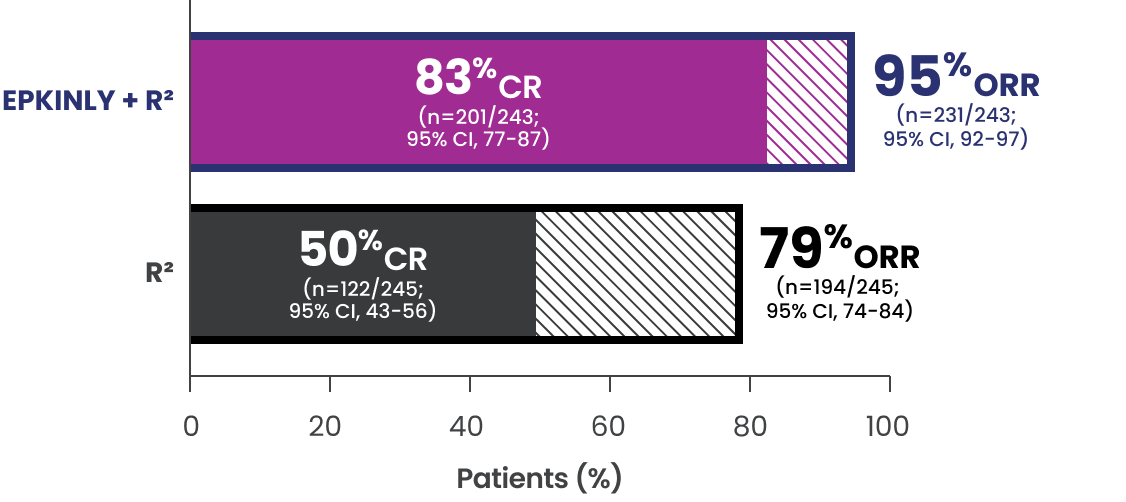
- The median duration of follow-up was 14.8 months in the ITT population
Data Limitation: No inference can be drawn from this data set. Follow-up analysis is exploratory, and data are descriptive in nature.
*Efficacy results determined by Lugano criteria (2014) as assessed by IRC and based on prespecified interim analysis.
†P-value is based on a prespecified analysis of the first 232 patients randomized.
‡P-value (one-sided) is from a Cochran-Mantel-Haenszel test stratified by disease history and region.
§95% CI is from the exact binomial distribution (Clopper-Pearson exact method).
EPKINLY + R2 provided sustained remission1-3*
IRC-assessed DOR at a median study follow-up of 14.8 months*†
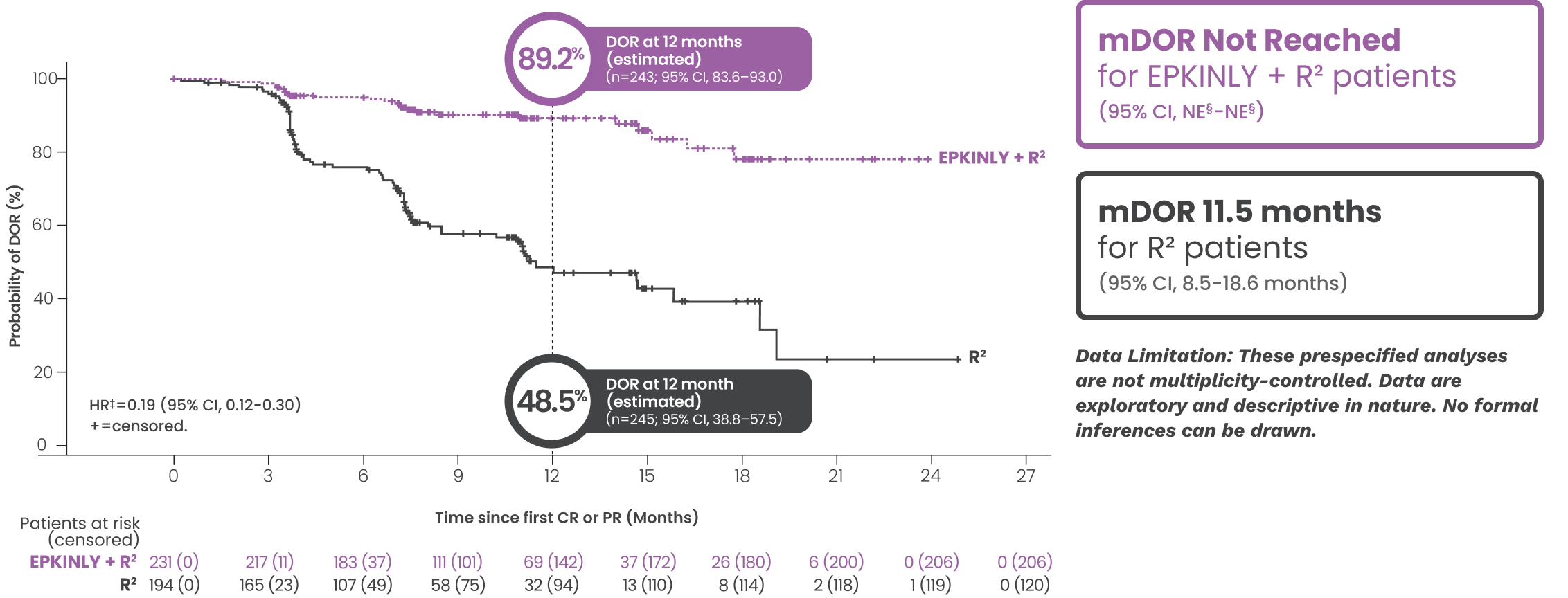
Date of analysis: May 24, 2025.
*Efficacy results, inclusive of PFS and DOR assessed progressive disease (PD), determined by Lugano criteria as assessed by IRC and based on a prespecified interim analysis.
†Based on Kaplan-Meier estimate.
‡Stratified by disease history/status. Hazard ratio is estimated using Cox proportional hazards model.
§Both lower and upper limits of the range indicate a censored value.
IRC-assessed DOCR at a median study follow-up of 14.8 months*†
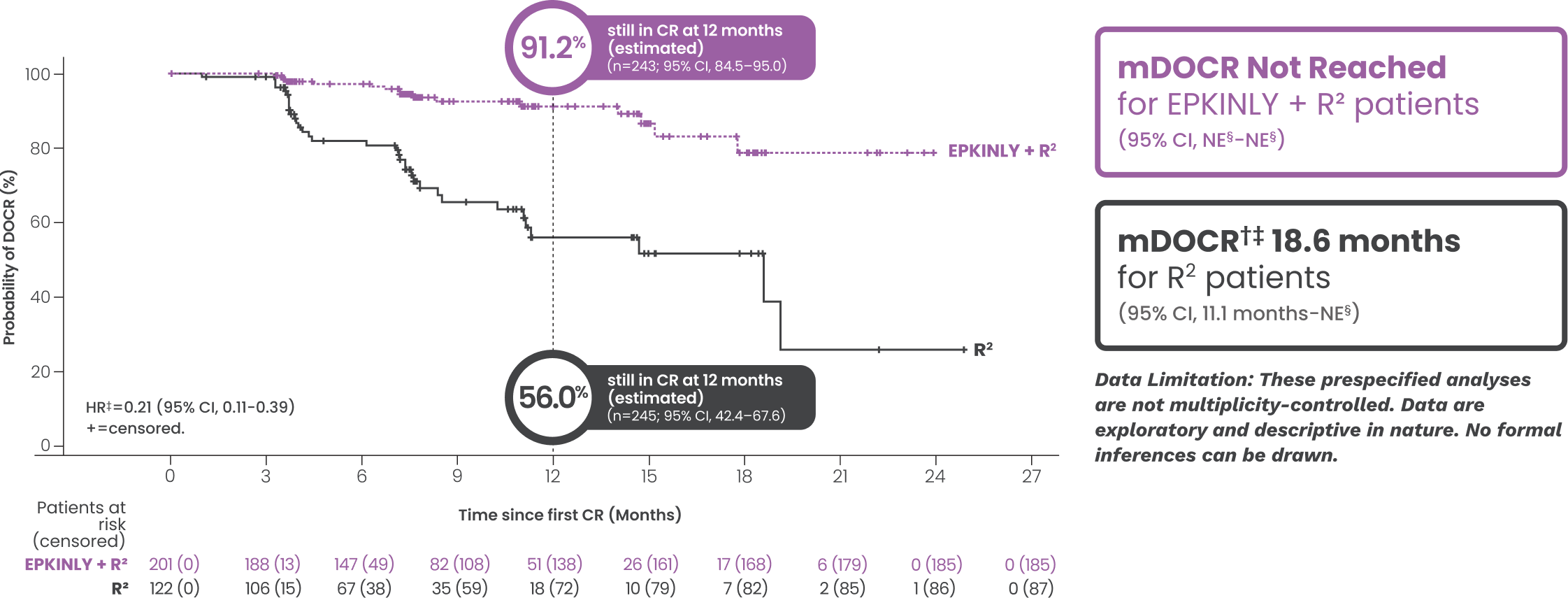
Date of analysis: May 24, 2025.
*Efficacy results, inclusive of PFS and DOR assessed progressive disease (PD), determined by Lugano criteria as assessed by IRC and based on a prespecified interim analysis.
†Based on Kaplan-Meier estimate.
‡Stratified by disease history/status. Hazard ratio is estimated using Cox proportional hazards model.
§Both lower and upper limits of the range indicate a censored value.
After 16 months, an estimated 93% of patients who received EPKINLY + R2 had not yet initiated any subsequent therapy, compared with 65% who received R2 *†
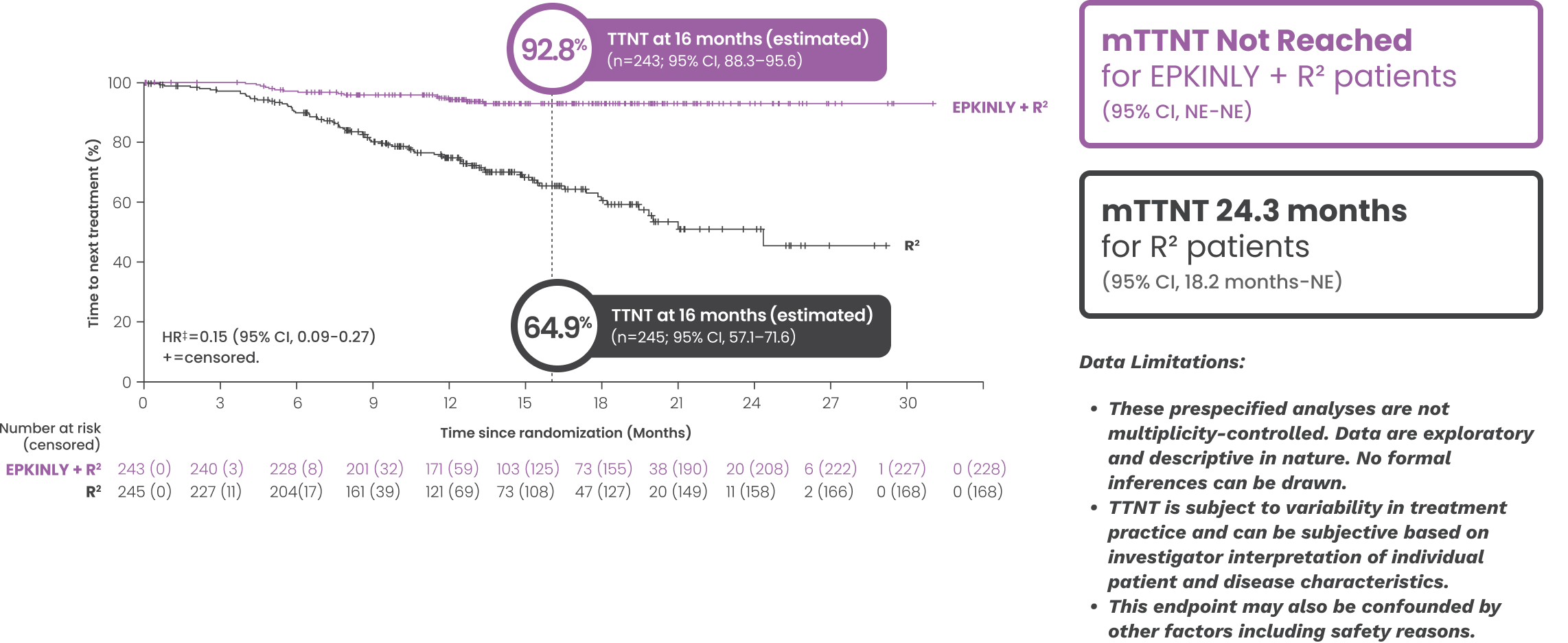
- The median duration of study follow-up was 14.8 months
Date of analysis: May 24, 2025.
*Efficacy results, inclusive of PFS and DOR assessed progressive disease (PD), determined by Lugano criteria as assessed by IRC and based on a prespecified interim analysis.
†Based on Kaplan-Meier estimate.
‡Stratified by disease history/status. Hazard ratio is estimated using Cox proportional hazards model.
2L=second line; CI=confidence interval; CR=complete response; HR=hazard ratio; IRC=Independent Review Committee; ITT=intent to treat; mDOCR=median duration of complete response; mDOR=median duration of response; NE=not evaluable; NR=not reached; ORR=overall response rate; PFS=progression-free survival; PR=partial response; R2=rituximab + lenalidomide; TTNT=time to next treatment.



