IMPORTANT SAFETY INFORMATION
BOXED WARNINGS
- Cytokine release syndrome (CRS), including serious or life-threatening reactions, can occur in patients receiving EPKINLY. Initiate treatment with the EPKINLY step-up dosage schedule to reduce the incidence and severity of CRS. Withhold EPKINLY until CRS resolves or permanently discontinue based on severity.
- Immune effector cell–associated neurotoxicity syndrome (ICANS), including life-threatening and fatal reactions, can occur with EPKINLY. Monitor patients for neurological signs or symptoms of ICANS during treatment. Withhold EPKINLY until ICANS resolves or permanently discontinue based on severity.
CRS
- CRS occurred in 51% of patients with large B-cell lymphoma (LBCL) in the clinical trial (37% grade 1, 17% grade 2, and 2.5% grade 3) and recurred in 16% of patients. Most events (92%) occurred during cycle 1, with 61% occurring after the 48 mg dose on cycle 1, day 15.
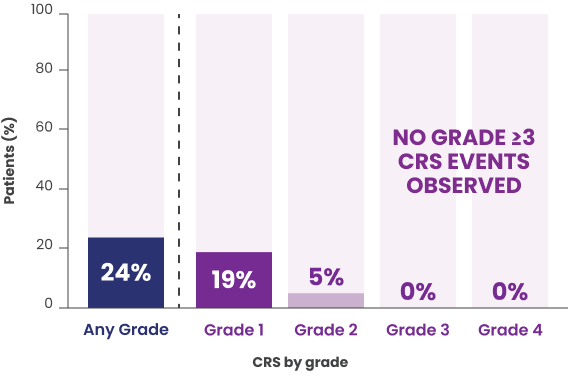
- CRS occurred in 49% of patients with FL receiving the recommended 3-step up dosage schedule in the clinical trial (45% grade 1, 9% grade 2) and recurred in 23% of patients. Most events (88%) occurred during cycle 1, with 49% occurring after the 48 mg dose on cycle 1, day 22.
- In patients who experienced CRS, the signs and symptoms included pyrexia, hypotension, hypoxia, dyspnea, chills, and tachycardia. Concurrent neurological adverse reactions associated with CRS occurred in 2.5% of patients with LBCL (reactions included headache, confusional state, tremors, dizziness, and ataxia) and 4.7% of patients with FL (reactions included headache and dizziness).
- Administer pretreatment medications to reduce the risk of CRS.
- Patients with DLBCL or high-grade B-cell lymphoma should be hospitalized for 24 hours following administration of the first full 48 mg dose.
- Monitor patients for potential CRS. At the first signs or symptoms of CRS, manage per current practice guidelines and administer supportive care as appropriate.
ICANS
- ICANS occurred in 6% of patients with LBCL in the clinical trial (4.5% grade 1, 1.3% grade 2, 0.6% fatal). Of the 10 ICANS events, 9 occurred in cycle 1 of treatment.
- ICANS occurred in 6% of patients with FL receiving the 2-step up dosage schedule in the clinical trial (3.9% grade 1, 2.4% grade 2).
- The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. Clinical manifestations of ICANS included, but were not limited to, confusional state, lethargy, tremor, dysgraphia, aphasia, and non-convulsive status epilepticus.
- Monitor patients for potential ICANS. At the first signs or symptoms of ICANS, manage per current practice guidelines and administer supportive care as appropriate.
Infections
- EPKINLY can cause serious and fatal infections. Serious infections, including opportunistic infections, were reported in 15% of patients with LBCL in the clinical trial (most common: 4.5% sepsis, 3.2% pneumonia). Fatal infections occurred in 1.3% of patients (1.3% COVID-19).
- Serious infections, including opportunistic infections, were reported in 40% of patients with FL receiving the 2-step up dosage schedule in the clinical trial (most common: 20% COVID-19, 13% pneumonia, 3% urinary tract infections). Fatal infections occurred in 6% of patients (5% COVID-19, 0.8% pneumonia, 0.8% sepsis).
- Monitor patients for signs and symptoms of infection prior to and during treatment and treat appropriately. Avoid administration in patients with active infections. Withhold or consider permanent discontinuation of EPKINLY based on severity. Prior to starting EPKINLY, provide Pneumocystis jirovecii pneumonia (PJP) prophylaxis and consider prophylaxis against herpes virus.
Cytopenias
- EPKINLY can cause serious or severe cytopenias. In the clinical trial of patients with LBCL, grade 3 or 4 events occurred in 32% (neutrophils decreased), 12% (hemoglobin decreased), and 12% (platelets decreased). Febrile neutropenia occurred in 2.5%.
- In the clinical trial of patients with FL receiving the 2-step up dosage schedule, grade 3 or 4 events occurred in 30% (neutrophils decreased), 10% (hemoglobin decreased), and 8% (platelets decreased). Febrile neutropenia occurred in 3.1%.
- Monitor complete blood counts throughout treatment. Based on severity of cytopenias, temporarily withhold or permanently discontinue EPKINLY. Consider prophylactic granulocyte colony-stimulating factor administration as applicable.
Embryo-Fetal Toxicity
- EPKINLY may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with EPKINLY and for 4 months after the last dose. Verify pregnancy status in females of reproductive potential prior to initiating EPKINLY.
Adverse Reactions
- DLBCL/HGBCL: Most common (≥20%) adverse reactions were CRS, fatigue, musculoskeletal pain, injection site reactions, pyrexia, abdominal pain, nausea, and diarrhea. Most common grade 3 to 4 laboratory abnormalities (≥10%) were decreased lymphocytes, decreased neutrophils, decreased white blood cells, decreased hemoglobin, and decreased platelets.
- FL: Most common (≥20%) adverse reactions were injection site reactions, CRS, COVID-19, fatigue, upper respiratory tract infection, musculoskeletal pain, rash, diarrhea, pyrexia, cough, and headache. The most common grade 3 to 4 laboratory abnormalities (≥10%) were decreased lymphocytes, decreased neutrophils, decreased white blood cells, and decreased hemoglobin.
Use in Specific Populations
- Lactation: Advise women not to breastfeed during treatment and for 4 months after the last dose of EPKINLY.
- Geriatric Use: In patients with relapsed or refractory FL who received EPKINLY in the clinical trial, 52% were ≥65 years old, and 13% were ≥75 years old. A higher rate of fatal adverse reactions, primarily infections, including COVID-19, was observed in patients ≥65 years old compared to younger adult patients. No overall difference in efficacy was observed.
INDICATIONS
EPKINLY is indicated for the treatment of adults with:
- relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS), including DLBCL arising from indolent lymphoma, and high-grade B-cell lymphoma (HGBCL) after 2 or more lines of systemic therapy.
- relapsed or refractory follicular lymphoma (FL) after 2 or more lines of systemic therapy.
These indications are approved under accelerated approval based on response rate and durability of response. Continued approval for these indications may be contingent upon verification and description of clinical benefit in confirmatory trials.
Please see full Prescribing Information, including Boxed Warnings.
05/2025 COM-US-EPK-0001501